Explanatory Note regarding Right to Quick Refund for Direct Purchase Trade Assurance Orders on Alibaba.com
I. Quick Refund:
Buyers are eligible to apply for a quick refund for any direct purchase Trade Assurance Orders on Alibaba.com within 2 hours of the buyers' successful payment excluding the scenario that supplier has already shipped. The system will provide an automatic refund to reduce buyers’waiting time, provided the amount of the Trade Assurance order is within the relevant limit and buyers are not required to negotiate with the seller.
II. Conditions and Process:
Eligible type of orders: Any direct purchase Trade Assurance order placed by buyers with an order amount not exceeding USD 200;
Usage: Buyers can apply for a quick refund of any direct purchase Trade Assurance order automatically generated by the system and without having to negotiate with the seller within 2 hours of the order being successfully paid by the buyer excluding the senario that supplier has already shipped goods as order requires.
Supported online payment methods: Credit/Debit Card, Online Transfer, Online Bank Payment, Apple pay, and Google pay
The announcement lose efficacy in Beijing time on July 15, 2021
[0416 Important Update] Supplementary Instructions on the Release of AliExpress Epidemic Prevention Materials and Commodities
Update date: 2020.4.16
Dear AliExpress merchants,
In order to meet the relevant legal and market supervision requirements, improve the overall product quality of AliExpress goods, optimize the buyer's shopping experience, and facilitate more merchants to understand the control requirements of the platform's epidemic prevention materials and commodities, the platform has explained and supplemented the release specifications of epidemic prevention materials and commodities again, as follows:
I. Product release category requirements
1. According to the effective rules, medical masks (belonging to medical devices) are available and can only be published in Health & Beauty / Health Care / Household Health Monitors / Medical Mas ks medical mask.
2. Non-medical protective masks should and should only be issued under the following four categories. It is strictly forbidden to publish protective masks under other categories except the following categories:
Health & Beauty / Health Care / Personal Health Care / Masks (non-medical) masks
Security & Protection / Workplace Safety Supplies / Masks / Protective Mask Mask
Security & Protection / Workplace Safety Supplies / Masks / Dust Mask
Mother & Kids / Baby Care / Baby Health Care / Baby Protective Mask Baby Protective Mask
3. Key tips: It is forbidden to release adult masks under infant masks, otherwise they will be punished according to category misplacement!
II. Product release specifications
1. Medical Masks
1) When the merchant releases Medical Masks, it is necessary to fill in or upload the commodity qualification information in the product release link. According to the different target market, fill in or upload the corresponding commodity qualification information, as follows:
· Medical masks made in China must obtain the domestic Class II device registration number (compulsory)
· Medical masks sold to the EU market need to obtain CE certification (optional)
· Medical masks sold to the U.S. market need to be registered with FDA (optional)
· Medical masks sold to the Korean market need to be registered as MFDS (optional)
2) When the merchant releases medical masks, the following statement should be added to the product details page:
Please be kindly noted:
· Listed product is produced and distributed abroad and subject to the laws of its country of origin .
· You may purchase the products for personal use only.
3) Frequently Asked Questions about Qualification Submission (FAQ): https://sell.aliexpress.com/zh/__pc/hQEeddqXpp.htm
2. Non-medical protective masks
1) For non-medical ordinary protective masks, do not publish them in the Medical Masks category, do not describe any medical use, and should choose the origin, safety standards, protection level and other attributes of masks according to the actual situation of the product. When the protective mask is released, the relevant protective level attributes must be checked when releasing the product, and the corresponding commodity qualification information must be uploaded, as follows:
· Claim that KN90/95/100 level protective masks need to obtain GB2626 standard test reports issued by CNAS-accredited laboratories (optional)
· Claim that FFP 1/2/3 grade or protective masks sold to the EU market need to obtain CE certification (optional)
· Claim that N95/99/100, R95, P95/99/100 levels of protective masks need to obtain a test report issued by NIOSH (optional)
· Claim that KF94-grade protective masks need to obtain a test report that meets the KS M 6673 standard of South Korea (optional)
2) When merchants release non-medical ordinary protective masks, they should add the following statement on the product details page:
Please be kindly noted, the listed product is produced and distributed abroad and subject to the laws of its country of origin.
3. Other release notes
1) It is forbidden to provide non-compliant medical/anti-virus goods. Merchants sell epidemic prevention goods. It is necessary to ensure legal sources, quality assurance, and consumer experience during special periods.
2) Merchants are prohibited from publishing and selling any products with direct text descriptions or implied semantics of the novel coronavirus (COVID-19). That is, merchants shall not publish novel coronavirus-containing text (including any language) anywhere on the platform, such as product information (including but not limited to product titles, rules, attributes, pictures, details, etc.), store decoration information (including but not limited to banner, store introduction, contact information, etc.) and its deformed words, abbreviations, abbreviations, code names, pictures and videos. At the same time, merchants shall not display any description or publicity that can detect, prevent, treat, cure or immunize the novel coronavirus in the text, pictures and videos of the products and stores released, and it is also forbidden to exaggerate/falsely describe the relevant standards and effects of epidemic prevention materials products. Examples: Antivirus, virus, Coronavirus, COVID-19, 2019-nCoV, SARS-CoV-2, etc. contain discriminatory statements that link the novel coronavirus epidemic or to region and race;
3) It is forbidden to mix ordinary masks and professional protective masks under the same product link, and the platform will strengthen its efforts to punish such SKU cheating;
4) Ordinary masks, that is, masks without protection level, professional protection level attributes shall be added when releasing goods, and medical use shall not appear in the description of goods.
and description of related protection levels such as N95/99/100, KN90/95/100, FFP1/2/3, etc.;
5) It is forbidden to sell masks, thermometers, oxygen generators, blood oxygen meters, ventilators, blood glucose meters, hand sanitizers/disinfectants, protective clothing, goggles, gloves, wipes, protective clothing, goggles, protective masks and other related epidemic prevention commodities in an unfair competitive way to disrupt market order, including but not limited to ultra-low or ultra-high prices. case;
6) It is forbidden to use epidemic-related information to introduce or recommend goods, including but not limited to publishing masks or other related epidemic prevention products for promotion;
III. Violations and Penalty Instructions
1. If the goods do not meet the requirements of the product release specifications, it is found that the platform will remove the goods that do not meet the requirements from the shelves for the first time; if the violation is violated again, the platform will delete the goods. If the violation is serious, the platform will directly delete the goods that do not meet the requirements;
2. The commodity category is misplaced, and the punishment methods are as follows: medical masks that are not placed in the prescribed medical mask category can be edited for the first time, and the goods can be deleted twice.
3. If the goods involve fraud of qualifications, the platform will punish illegal goods and stores, including but not limited to (i) returning or deleting goods/information; (ii) restricting the release of goods; (iii) temporarily freezing accounts and account balances; and (iv) closing accounts. For users who close their accounts, AliExpress has the right to take measures to prevent the user from registering on AliExpress again.
Warm reminder: It is recommended that merchants verify the commodity qualification information before releasing the goods to avoid the punishment of goods due to non-conformity of qualifications. The official public database that can be referred to includes but is not limited to:
1) List of EU CE certification bodies:
https://ec.europa.eu/growth/tools-databases/nando/index.cfm? fuseaction=notifiedbody.main,
And ensure that the agency has the scope of authorization (such as Medical Device Directive 93/42/EEC Medical Devices, Personal Protective Equipment Regulation (EU) 2016/425 Personal prote) ctive equipment)
2) U.S. FDA's equipment product database: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
3) Registration/recording of medical devices in China:
4) CNAS-approved mask testing institutions: https://www.cnas.org.cn/english/photonews/03/902316.shtml
5) N95 mask list approved by Niosh: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html
IV. Other instructions
For the delivery specifications and performance assessment instructions of epidemic prevention materials, please refer to the content of the announcement: (0331 Important Update) Announcement on the Control and Upgrade of AliExpress Epidemic Prevention Materials
For accounts that do not follow the platform's specifications and have serious violations, penalties shall be imposed in accordance with AliExpress's "Severe Disrupt Platform Order" Rules:
Serious: 12 minutes/time (7 days of account freeze)
Particularly serious: 48 points/time (close the account)
For more information about the platform's penalties for disrupting the order of the platform, you can check the relevant rules:
Analysis and Punishment Rules for Search Cheating Cases
AliExpress's "Severate Disruption of Platform Order" Rules
For the announcement content of the platform's epidemic information, you can view: about the overseas epidemic-related nationalized sales ban and restriction policies (continuous update)
In this special period, thank you for your understanding and continuous support and trust in AliExpress.
AliExpress
2020.4.16
[0424 Important Update] Instructions on Violations of Epidemic Prevention Materials and Punishment Upgrades
Dear AliExpress merchants,
With the continuous spread of the epidemic overseas, the national control policy of epidemic prevention materials, especially medical epidemic prevention materials, is also constantly upgraded and changing. The platform fully supports and guarantees the normal and compliant operation of merchants, and continuously improves the overall product quality of AliExpress goods and optimizes the buyer's shopping experience. However, we have also recently also found that some merchants There are serious disturbances of the market order in the home, including violating or maliciously circumventing the platform's epidemic prevention material release category specifications (i.e. category misplacement, etc.), exaggerating/falsely describing the relevant qualification standards of epidemic prevention material products (such as CE/FDA/NIOSH certification, etc.) and protection levels (such as KN95/N95/F) FP2, etc.), these behaviors seriously violate the rules of the platform, and also seriously harm the rights and interests and experience of consumers. I would like to remind all merchants to operate legally and abide by the rules of the platform. In the near future, the platform will strictly screen and upgrade the above behaviors, including but not limited to:
1. If the goods do not meet the product release specifications, the platform will directly delete the illegal goods and deduct 2 points;
2. If the goods do not meet the requirements of product release category, the platform will directly delete the illegal goods and deduct 2 points;
3. If the goods involve fraud of qualifications, the platform will directly delete the illegal goods and deduct 6 points; at the same time, the penalties for the store include but are not limited to 1) restricting the release of goods; 2) temporarily freezing the account and account balance; 3) closing the account. For users who close their accounts, AliExpress reserves the right to take measures to prevent the user from registering on AliExpress again.
4. For accounts that do not follow the platform's specifications and have serious violations, penalties shall be imposed in accordance with AliExpress's "Severe Disrupt the Order of the Platform' Rules": serious: 12 minutes/time (7 days of account freezing), especially serious: 48 minutes/time (close the account)
The platform will start rectification on April 27. Please do a good job in self-inspection and self-inspection to avoid affecting the normal operation and flow of the store, and avoid deductions, freezing or other penalties due to store commodity problems.
For more shipping specifications for epidemic prevention materials, please refer to the content of the announcement: (0416 Important Update) Supplementary Instructions on the Issuance Specifications for AliExpress Epidemic Prevention Materials and Commodities;
Please also pay close attention to the page of "Overseas Epidemic-related Nationalized Prohibition and Restriction Policy (Continuous Update)": https://sell.aliexpress.com/4sxYf5QoRL.htm Learn about the relevant policies and we will continue to update.
For more information about the platform's penalties for disrupting the order of the platform, you can check the relevant rules:
Analysis and Punishment Rules for Search Cheating Cases
AliExpress's "Severate Disruption of Platform Order" Rules
In this special period, thank you for your understanding and continuous support and trust in AliExpress.
FAQ
I. Under what circumstances will be punished and deducted 2 points?
1. Category staggered category (take masks as an example)
a. Protective masks such as N95, N99, KN90, KN95, KN100, KF94, KF99, FFP1, FFP2, FFP3 are not medical masks, but must be published under the following corresponding mask categories: (Masks (None medical) masks (non- Medical) / Protective Mask Mask / Dust Mask Dust Mask / Baby Mask Infant Mask), otherwise the category is misplaced.
b. Products under non-medical categories use medical terms, such as medical (product information does not mention NonMedical, Non-Medical), medical-related descriptions such as Surgical, dental, doctor, hospital, etc. Otherwise, the category is misplaced.
c. Other categories sell masks in staggered ways, such as actual sales of masks under unrelated categories.
2. Exaggerated publicity (take masks as an example)
a. The protection level is claimed but the corresponding test report certificate is not provided. For example, the N95 test report certificate of N95 is not provided, and it is submitted in the "qualification information" section of the commodity release interface.
b. Multiple protection levels have been declared but no complete test report certificates have been provided, such as N95, KN95 and FFP2, but only one certificate has been provided. The corresponding qualification certificate must be submitted in the "Qualification Information" section according to the declared protection level.
c. Claimed to have CE/FDA certified but did not provide the corresponding certificate, submitted in the "Qualification Information" section. ( Attached is how to inquire about the validity of CE certificates:
FDA qualification is a screenshot of the medical device registration page included in the official website, which is a screenshot, not a certificate.
II. Under what circumstances will 6 points be deducted?
Answer: Take masks as an example. In the "Qualification Information" column of the product release page or the main picture or product details page, as long as other people's certificates are fraudulently used or the CE, FDA, NIOSH and other licenses provided have been verified as invalid certificates, 6 points will be punished and deducted. It is important to know the seriousness. Before you claim and submit the relevant certification, you must verify that the information is true and valid.
Warm reminder: It is recommended that merchants verify the commodity qualification information before releasing the goods to avoid the punishment of goods due to non-conformity of qualifications. The official public database that can be referred to includes but is not limited to:
1) List of EU CE certification bodies:
https://ec.europa.eu/growth/tools-databases/nando/index.cfm? fuseaction=notifiedbody.main,
And ensure that the agency has the scope of authorization (such as Medical Device Directive 93/42/EEC Medical Devices, Personal Protective Equipment Regulation (EU) 2016/425 Personal prote) ctive equipment)
2) U.S. FDA's equipment product database: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
FDA qualification is a screenshot of the medical device registration page included in the official website, which is a screenshot, not a certificate.
3) Registration/recording of medical devices in China:
4) CNAS-approved mask testing institutions: https://www.cnas.org.cn/english/photonews/03/902316.shtml
5) N95 mask list approved by Niosh: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html
AliExpress
April 24, 2020
Dear AliExpress merchants,
During the epidemic, in order to ensure a better business environment for merchants and the performance of buyers' shopping, the platform has issued a series of control policies for epidemic prevention materials. At the same time, in order to meet relevant laws and market supervision requirements, the platform reiterates the following for the delivery and performance assessment of goods of epidemic prevention materials:
I. Upgrading of stocking time and delivery logistics lines
During the epidemic, in order to provide better logistics guarantee and shopping experience, the platform will make the following policy descriptions and adjustments:
1. The order preparation period for epidemic prevention materials has been adjusted from 7 working days (see the platform's "Announcement on the Control and Upgrade of AliExpress Epidemic Prevention Materials" on March 31) to 72 hours. Considering the provision of merchant transition period, the platform is actually adjusted to 3 working days.
From May 6, 2020, Beijing time, the order preparation period of epidemic prevention materials will be adjusted to 72 hours, that is, the merchant fails to complete the shipment within 72 hours after the consumer order is successfully paid. The order will be closed by the system overtime, and the payment will be returned to the buyer. The order status shows that the order is closed, and the order will be counted. If you enter the transaction and don't sell it, it is recommended that merchants pay attention to the timeliness of delivery in the background;
2. In order to comply with the Announcement No. 53 of 2020 of the General Administration of Customs and the Announcement of the State Administration of Market Supervision and Administration of the Ministry of Commerce No. 12 of 2020, the customs needs export sellers to provide export compliance statements for goods, involving 10 categories on the platform. The export declaration of medical supplies and logistics upgrade requirements are as follows:
1) Merchants and commodities under the 10 categories shall upload the corresponding export statement of medical supplies at the time of release. Please tell the truthfully. The breakdown of the category is as follows:
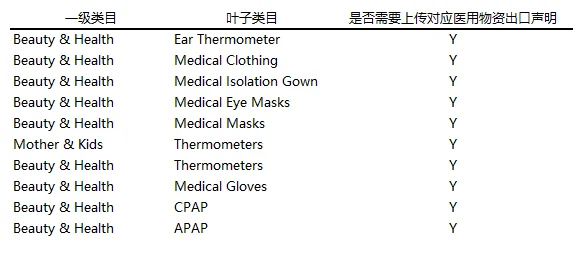
2) Statement of Export Medical Supplies (Chinese and English) (Please download Annex 2 of the announcement):
http://www.mofcom.gov.cn/article/ae/ai/202004/20200402958970.shtml
Statement on Export of Medical Supplies (Chinese and English) Fill in the specifications, reference link:
3) Upload entrance: the qualification module of the product release page (under the attribute bar)
Frequently Asked Questions about Qualification Submission (FAQ): https://sell.aliexpress.com/zh/__pc/hQEeddqXpp.htm
4) Entry effective time: Estimated April 30, 2020 Beijing time
The platform will implement control on May 15. If the goods are not uploaded to the medical material export statement or the uploaded statement is not in compliance, it is found for the first time that the platform will remove the goods that do not meet the requirements from the shelves; if the violation is violated again, the platform will delete the goods; if the violation is serious, whether it is found for the first time or not, The platform will delete goods that do not meet the requirements directly and deduct 2 points. Note: This part is stated not to be displayed to consumers, but only provided to your logistics service providers and customs to complete rapid customs clearance verification.
3. Due to the special restrictions on customs and logistics in various countries during the epidemic, the platform will offline economical, simple and customized logistics lines for 29 categories, that is, epidemic-related categories can only be shipped through AliExpress worry-free logistics-standard lines during the overseas epidemic, some of which are liquid, The sensitive goods with tape still retain the permission to customize the category. Please adjust the logistics freight template under the corresponding category before the line is offline. The estimated effective time is May 6, 2020, Beijing time. It is recommended that the merchant pay attention to the background in time. Please refer to the following table for relevant categories and line closure plans:
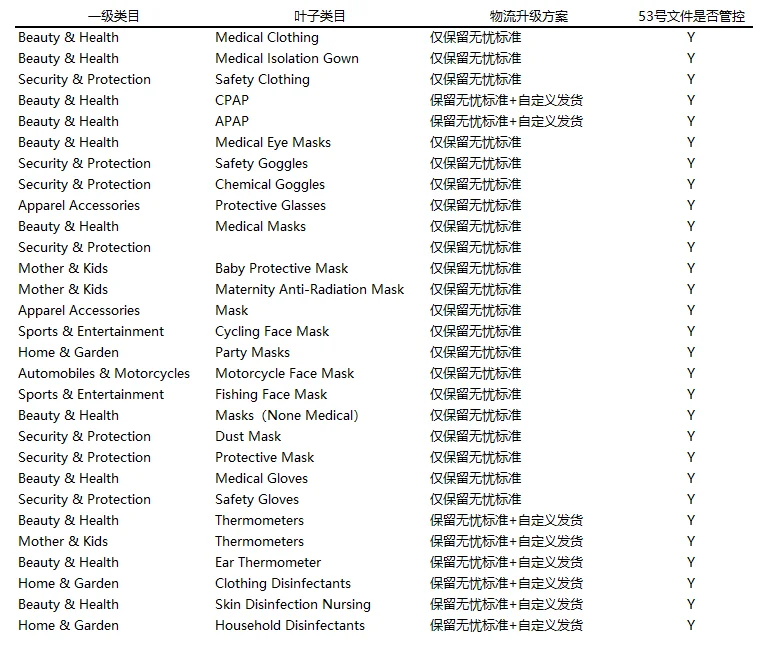
Note: If the corresponding product has signed up for the platform activity, the activity locks the previous freight template, and you have only set up economical, simple and customized lines before. The offline of these lines will cause your goods to be unable to place an order to the corresponding country during the event. In this situation, please provide the product ID, activity name, and activity I D Contact Xiaohe Online Customer Service to submit an exit application for the activity.
You can enter the marketing campaign - platform activity - view all activities - view the activities to be confirmed and participating, and the platform activities displayed after May 6 will be affected.
II. For the performance assessment instructions of epidemic prevention materials and commodities, please refer to the content of the announcement: (0331 Important Update) Announcement on the Control and Upgrade of AliExpress Epidemic Prevention Materials and Commodities
- Other instructions
For accounts that do not follow the platform's specifications and have serious violations, penalties shall be imposed in accordance with AliExpress's "Severe Disrupt Platform Order" Rules:
Serious: 12 minutes/time (7 days of account freeze)
Particularly serious: 48 points/time (close the account)
For more information about the platform's penalties for disrupting the order of the platform, you can check the relevant rules:
Analysis and Punishment Rules for Search Cheating Cases
AliExpress's "Severate Disruption of Platform Order" Rules
For the announcement content of the platform's epidemic information, you can view: about the overseas epidemic-related nationalized sales ban and restriction policies (continuous update)
In this special period, thank you for your understanding and continuous support and trust in AliExpress.
AliExpress
2020.04.30
[0428 Important Update] Supplementary Instructions on the Control of AliExpress Epidemic Prevention Materials
Dear AliExpress merchants,
In order to meet the relevant legal and market supervision requirements, improve the overall product quality of AliExpress goods, optimize the buyer's shopping experience, and facilitate merchants to better understand the control requirements of the platform's epidemic prevention materials and commodities, the platform's release norms and penalties for epidemic prevention materials and violations are further explained as follows:
I. Standard for the issuance of epidemic prevention materials and commodities:
- Requirements for the release of medical products
1. Medical masks (belonging to medical devices) are available and can only be published in Health & Beauty / Health Care / Household Health Monitors / Medical Masks.
2. Medical goggles/eye masks have and can only publish Beauty & Health/Health Care/Household Health Monitors/Medical Eye Masks medical eye masks
2. Medical protective clothing is available and can only be published in Beauty & Health/Health Care/HouseholdHealth Monitors/Medical Clothing Medical Protective Clothing
3. Medical protective clothing isolation clothing, surgical caps, surgical clothing, surgical shoe covers and other medical surgical-related clothing can only be published in Beauty & Health/Health Care/Household Health Monitors/Medica l Isolation Gown medical isolation suit
4. Infrared human thermometers (intelligent thermometers, frontal thermometers) can only be published in Beauty & Health/Health Care/Household Health Monitors/Thermometers thermometers
5. Ear temperature guns are available and can only be published in Beauty & Health/Health Care/Household Health Monitors/Ear Thermometer Ear Temperature Guns
6. Children's infrared thermometers (smart thermometers, frontal thermometers, ear thermometers) are available and can only be published in Mother & Kids/Baby Care/Baby Health Care/Thermometers thermometers
7. Medical gloves are available and can only be published in Beauty & Health/Health Care/Household Health Monitors/Medical Gloves medical gloves
8. ECG monitors are available and can only be published in Beauty & Health/Health Care/Household Health Monitors/Electrocardioscanner
9. Medical disinfection care products are available and can only be published in Beauty & Health/Health Care/Household Health Monitors/ Skin Disinfection Nursing Skin Disinfection care
10. Oxygen machines have and can only be published in
Beauty & Health/Health Care/Household Health Monitors/Oxygen Machine Oxygen Machine
11. The blood oxygen meter has and can only be published in
Beauty & Health/Health Care /Household Health Monitors/Oximetry Blood Oxygen Meter
12. Ventilators have and can only be published in the corresponding categories below
Beauty & Health/Health Care Household Health Monitors/CPAP Continuous Positive Pressure Ventilator
Beauty & Health/Health Care /Household Health Monitors APAP Automatic Airway Positive Pressure Ventilator
- Requirements for the release of other epidemic prevention commodities
1. Non-medical protective masks should and should only be issued under the following four categories. It is strictly forbidden to publish protective masks under other categories other than the following categories:
Health & Beauty / Health Care / Personal Health Care / Masks (non-medical) masks
Security & Protection / Workplace Safety Supplies / Masks / Protective Mask Mask
Security & Protection / Workplace Safety Supplies / Masks / Dust Mask
Mother & Kids / Baby Care / Baby Health Care / Baby Protective Mask Baby Protective Mask
Key Tip: It is forbidden to release adult masks under infant masks, otherwise they will be punished according to category misplacement!
2. Non-medical goggles/eye masks are published to the following corresponding categories according to category ownership
Published in Security & Protection > Workplace Safety Supplies > Chemical Goggles Chemical Safety Goggles
Security & Protection > Workplace Safety Supplies > Safety Goggles Eye Mask
Apparel Accessories > Eyewear & Accessories > Protective glasses goggles (Led, ray goggles)
3. Non-medical protective clothing/isolation clothing, uniformly released to Security & Protection > Workplace Safety Supplies > Safety Clothing safety protective clothing
4. Non-medical protective gloves, uniformly released to Security & Protection > Workplace Safety Supplies > Safety Gloves protective gloves
5. Please publish hand sanitizer according to the category under the following corresponding categories.
Beauty & Health/Skin Care/Hands & Nails/Hand Soaps Hand Sanitizer
Mother & Kids/Pregnancy &/Maternity/Skin Care Maternity Hand Sanitizer
6. Disinfection goods are published under the following corresponding categories according to the category
Home & Garden/Household Merchandises/House Cleaning Tools & Accessories/ Disinfectant Wipes Kitchen Disinfection wipes
Home & Garden/Household Merchandises/Household Cleaning Chemicals/Clothing Disinfectants Clothing Disinfectants
Home & Garden/Household Merchandises/Household Cleaning Chemicals/Household Disinfectants Home Disinfectants
II. Product release specifications
1. Medical Masks
1) When the merchant releases Medical Masks, it is necessary to fill in or upload the commodity qualification information in the product release link. According to the different target market, fill in or upload the corresponding commodity qualification information, as follows:
· Medical masks made in China must obtain the domestic Class II device registration number (compulsory)
· Medical masks sold to the EU market need to obtain CE certification (optional)
· Medical masks sold to the U.S. market need to be registered with FDA (optional)
· Medical masks sold to the Korean market need to be registered as MFDS (optional)
2) When the merchant releases medical masks, the following statement should be added to the product details page:
The products available to purchase on this page are manufactured and sold in compliance with the law s of China. If you are importing these goods, you should ensure that you comply with your country's laws and reg ulations.
You may purchase the products for personal use only.
If the goods sold by the merchant also comply with the laws, regulations or standards of the destination country (such as EU FFP 2), the following statement should be added on the product details page (XXX should be changed to the name of the destination country):
The products available to purchase on this page are manufactured and sold in compliance with the law s of China and XXX. If you are importing these goods, you should ensure that you comply with your country's laws and reg ulations.
You may purchase the products for personal use only.
Please be kindly noted:
· Listed product is produced and distributed abroad and subject to the laws of its country of origin .
· You may purchase the products for personal use only.
3) Frequently Asked Questions about Qualification Submission (FAQ): https://sell.aliexpress.com/zh/__pc/hQEeddqXpp.htm
2. Non-medical protective masks
1) For non-medical ordinary protective masks, do not publish them in the Medical Masks category, do not describe any medical use, and should choose the origin, safety standards, protection level and other attributes of masks according to the actual situation of the product. When the protective mask is released, the relevant protective level attributes must be checked and the corresponding commodity qualification information must be uploaded, as follows:
· Claim that KN90/95/100 level protective masks need to obtain GB2626 standard test reports issued by CNAS-accredited laboratories (optional)
· Claim that FFP 1/2/3 grade or protective masks sold to the EU market need to obtain CE certification (optional)
· CLAIM THAT N95/99/100, R95, P95/99/100 LEVELS OF PROTECTIVE MASKS NEED TO OBTAIN A TEST REPORT ISSUED BY NOISH (OPTIONAL)
· Claim that KF94-grade protective masks need to obtain a test report that meets the KS M 6673 standard of South Korea (optional)
2) When merchants release non-medical ordinary protective masks, they should add the following statement on the product details page:
The products available to purchase on this page are manufactured and sold in compliance with the law s of China. If you are importing these goods, you should ensure that you comply with your country's laws and reg ulations.
If the goods sold by the merchant also comply with the laws, regulations or standards of the destination country (such as EU FFP 2), the following statement should be added on the product details page (XXX should be changed to the name of the destination country):
The products available to purchase on this page are manufactured and sold in compliance with the law s of China and XXX. If you are importing these goods, you should ensure that you comply with your country's laws and reg ulations.
3. Other release notes
1) It is forbidden to provide non-compliant medical/anti-virus goods. Merchants sell epidemic prevention goods. It is necessary to ensure legal sources, quality assurance, and consumer experience during special periods.
2) Merchants are prohibited from publishing and selling any products with direct text descriptions or implied semantics of the novel coronavirus (COVID-19). That is, merchants shall not publish text containing or impliciting the novel coronavirus (including any) in any place on the platform, such as product information (including but not limited to product title, rules, attributes, pictures, details, etc.), store decoration information (including but not limited to banner, store introduction, contact information, etc.), etc. He language) and its deformation words, abbreviations, abbreviations, code names of text, pictures and videos. At the same time, merchants shall not display any descriptions, publicity or hints that can detect, prevent, treat, cure or immunize the novel coronavirus in the text, pictures and videos of the products and stores released, and it is also forbidden to exaggerate/falsely describe the relevant standards and effects of epidemic prevention materials products. Examples of violations: Antivirus, virus, Coronavirus, COVID-19, 2019-nCoV, SARS-CoV-2, etc. contain discriminatory statements that link the novel coronavirus epidemic or region or race;
3) It is forbidden to mix ordinary masks and professional protective masks under the same product link, and the platform will strengthen its efforts to punish such SKU cheating;
4) Ordinary masks, that is, masks without protection level, professional protection level attributes shall be added when releasing goods, and medical use shall not appear in the description of goods.
and description of related protection levels such as N95/99/100, KN90/95/100, FFP1/2/3, etc.;
5) Masks, thermometers, oxygen generators, blood oxygen meters, ventilators, blood glucose meters, hand sanitizers/disinfectants, protective clothing, goggles, gloves, wipes, protective clothing, goggles, protective masks, UV lamps and other related epidemic prevention commodities are prohibited from being sold in an unfair competitive way to disrupt market order, including but not limited to Low or ultra-high price sales;
6) It is forbidden to use epidemic-related information to introduce or recommend goods, including but not limited to publishing masks or other related epidemic prevention products for promotion;
7) The product photo must show the label or the content of the label must be seen by the buyer in other ways, in order to let the buyer know what the characteristics of the product are (such as about disinfection gel goods);
8) As a seller, you are responsible for ensuring that the goods you provide comply with the relevant laws and regulations of the country of sale. If the merchant suspects that the goods may not meet the compliance requirements of the target country, the merchant must remind the buyer that the goods can only comply with the merchant's national laws and regulations, and may not necessarily comply with the buyer's national laws and regulations;
III. Violations and Penalty Instructions
1. If the goods do not meet the product release specifications, the platform will directly delete the illegal goods and deduct 2 points;
2. If the goods do not meet the requirements of product release category, the platform will directly delete the illegal goods and deduct 2 points;
3. If the goods involve fraud of qualifications, the platform will directly delete the illegal goods and deduct 6 points; at the same time, the penalties for the store include but are not limited to 1) restricting the release of goods; 2) temporarily freezing the account and account balance; 3) closing the account. For users who close their accounts, AliExpress reserves the right to take measures to prevent the user from registering on AliExpress again.
4. For accounts that do not follow the platform's specifications and have serious violations, penalties shall be imposed in accordance with AliExpress's "Severe Disrupt the Order of the Platform' Rules": serious: 12 minutes/time (7 days of account freezing), especially serious: 48 minutes/time (close the account)
The platform has started rectification on April 27. Please do a good job in self-inspection and self-inspection to avoid affecting the normal operation and flow of the store, and avoid deductions, freezing or other penalties due to store commodity problems. For details, please refer to the "0424 Important Update] Instructions for Violations of Epidemic Prevention Materials and Punishment Upgrades.
https://sell.aliexpress.com/zh/__pc/b0tlqeuNS5.htm? spm=5261.ams_97443.0.0.1f8asByIsByIJo
Warm reminder: It is recommended that merchants verify the commodity qualification information before releasing the goods to avoid the punishment of goods due to non-conformity of qualifications. The official public database that can be referred to includes but is not limited to:
1) List of EU CE certification bodies:
https://ec.europa.eu/growth/tools-databases/nando/index.cfm? fuseaction=notifiedbody.main,
And ensure that the agency has the scope of authorization (such as Medical Device Directive 93/42/EEC Medical Devices, Personal Protective Equipment Regulation (EU) 2016/425 Personal prote) ctive equipment)
2) U.S. FDA's equipment product database: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm
3) Registration/recording of medical devices in China:
4) CNAS-approved mask testing institutions: https://www.cnas.org.cn/english/photonews/03/902316.shtml
5) N95 mask list approved by Niosh: https://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/n95list1.html
Other instructions
For accounts that do not follow the platform's specifications and have serious violations, penalties shall be imposed in accordance with AliExpress's "Severe Disrupt Platform Order" Rules:
Serious: 12 minutes/time (7 days of account freeze)
Particularly serious: 48 points/time (close the account)
For more information about the platform's penalties for disrupting the order of the platform, you can check the relevant rules:
Analysis and Punishment Rules for Search Cheating Cases
AliExpress's "Severate Disruption of Platform Order" Rules
For the announcement content of the platform's epidemic information, you can view: about the overseas epidemic-related nationalized sales ban and restriction policies (continuous update)
In this special period, thank you for your understanding and continuous support and trust in AliExpress.
AliExpress
April 28, 2020
Comments
Post a Comment